The EPP Group wants the European Union to become what it calls a true Health Union with more health powers. To this end, the EPP Group will vote this week for more resources for the European Medicines Agency (EMA) to prevent shortages of critical medicines and medicinal devices. Furthermore, clinical trials will be made more…
Healthcare
Titanium Dioxide Banned As Food Additive
The European Commission has adopted a ban on the use of Titanium Dioxide as a food additive (E171). The ban will apply after a six-month transitory period. This means that, as from this summer, this additive should no longer be added to food products. Commissioner Stella Kyriakides, in charge of Health and Food Safety, said: “The safety of the…
Impact of Coronavirus on Work-Life Balance
The Covid-19 pandemic clearly shows that many of the Work-Life Balance Directive 2019 provisions – to be implemented by Member States by August this year – are insufficient. The Covid-19 pandemic has exacerbated work-life balance conflicts for workers with care responsibilities, the majority of whom are women. For ETUI Researcher Kalina Arabadjieva, this new EU legal…
Protecting Workers Against Hazardous Substances
A revision of rules to protect workers of carcinogenic and other hazardous substances has been informally agreed by the European Parliament and Council negotiators. During the negotiations, MEPs secured the inclusion of reprotoxic substances in the fourth revision of the Carcinogens and Mutagens at Work Directive (CMD4). These substances have adverse effects on reproduction and…
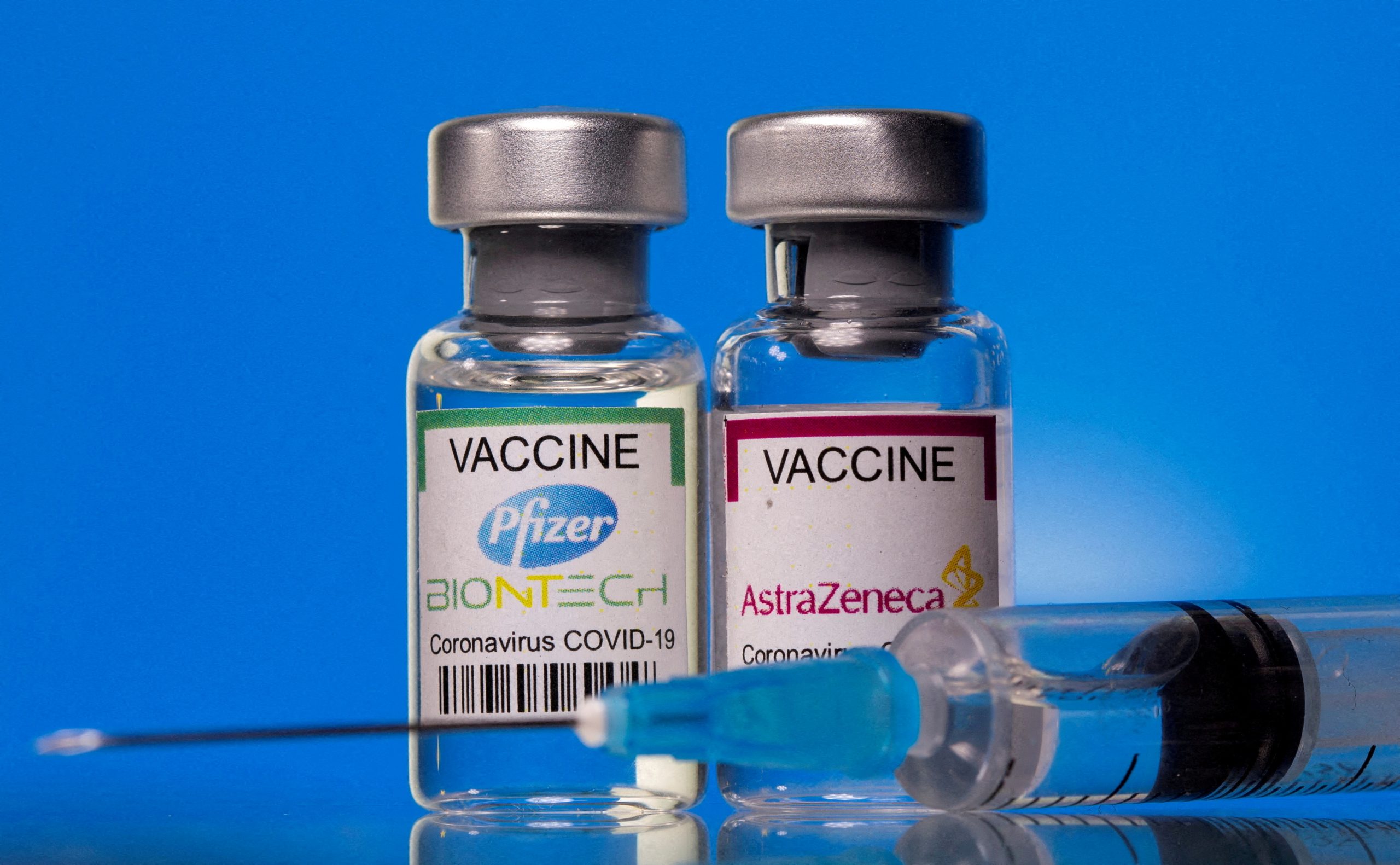
EU Authorises New Vaccine Nuvaxovid
The European Commission has granted a conditional marketing authorisation (CMA) for the COVID‑19 vaccine Nuvaxovid, developed by Novavax, the fifth COVID-19 vaccine authorised in the EU. This authorisation follows a positive scientific recommendation based on a thorough assessment of the safety, effectiveness and quality of the vaccine by the European Medicines Agency (EMA) and is…
EU Digital COVID 19 Certificate
The Commission has adopted rules relating to the EU Digital COVID Certificate, establishing a binding acceptance period of 9 months (precisely 270 days) of vaccination certificates for the purposes of intra-EU travel. A clear and uniform acceptance period for vaccination certificates will guarantee that travel measures continue to be coordinated, as called for by the European Council…
In Vitro Diagnostic Medical Devices Regulation
The In Vitro diagnostic medical devices regulation that will apply as from 26 May 2022, can now be progressively rolled out, thanks to its adoption by the European Parliament and the Council. In the context of the COVID-19 pandemic Member States, health institutions and economic operators redeployed financial and other resources to address the unprecedented…
BioNTech Pfizer Accelerates Vaccine Delivery
The Commission has today agreed with BioNtech-Pfizer to accelerate the delivery of its mRNA vaccine to Member States, starting in a few weeks. In Q1 of 2022, BioNtech-Pfizer will deliver an additional 20 million vaccine doses (5 million in January, 5 million in February and 10 million in March). These doses come on top of…
Supply of Medicines from UK
The Commission has put forward proposals to ensure the continued long-term supply of medicines from Great Britain to Northern Ireland and to address outstanding supply concerns in Cyprus, Ireland and Malta. In the context of the Protocol on Ireland/Northern Ireland, this means that the same medicines will continue to be available in Northern Ireland at…
Fighting the Scourge of Cancer
The ECR MEPs involved in the final report of the Special Committee on Beating Cancer, Shadow Rapporteur Pietro Fiocchi, and First Committee Vice-Chair and ECR Coordinator Joanna Kopcińska, say they are “broadly satisfied” with the direction taken by Parliament in this report in fighting the scourge of cancer. “We welcome the report’s focus on the key themes…