On World Mental Health day this year (10 October), Mental Health Europe hosted an event at the European Parliament focusing on refugees, migration and mental health. World Mental Health day comes and goes every year, complete largely with perfunctory nods from politicians towards “delivering”, “stakeholders” and “comprehensive solutions”. The proceedings organised by the European Parliament…
Tag: #Kyriakides
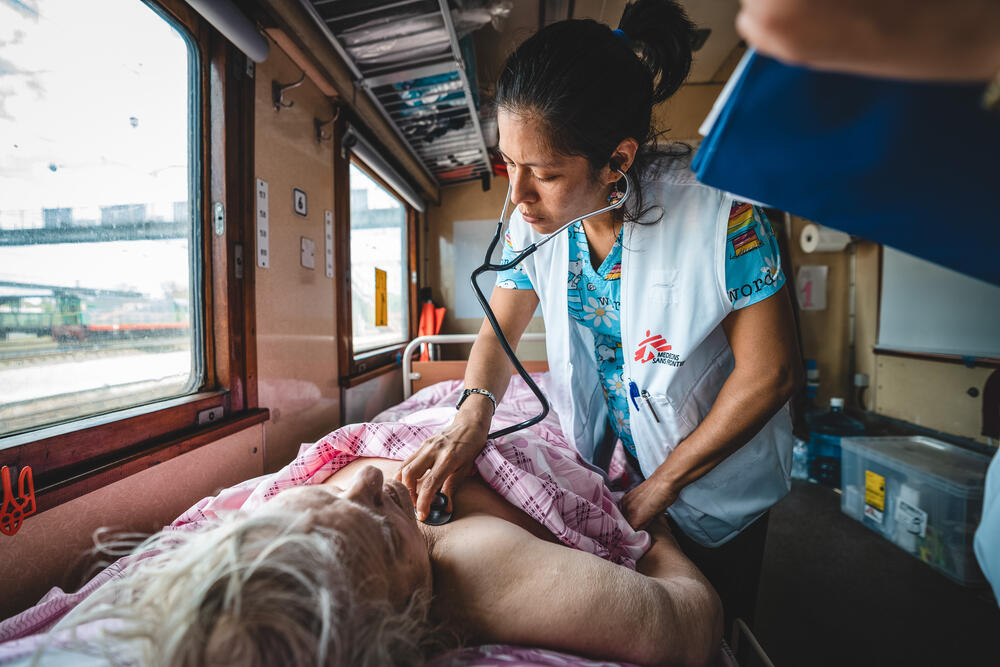
Medical Evacuations from Ukraine
The EU says it has successfully coordinated 1,000 medical evacuations of Ukrainian patients via its civil protection mechanism to provide them with specialised healthcare in hospitals across Europe. As the number of wounded people in Ukraine increases day by day, local hospitals are struggling to keep up with the demand. At the same time, Poland, Moldova…
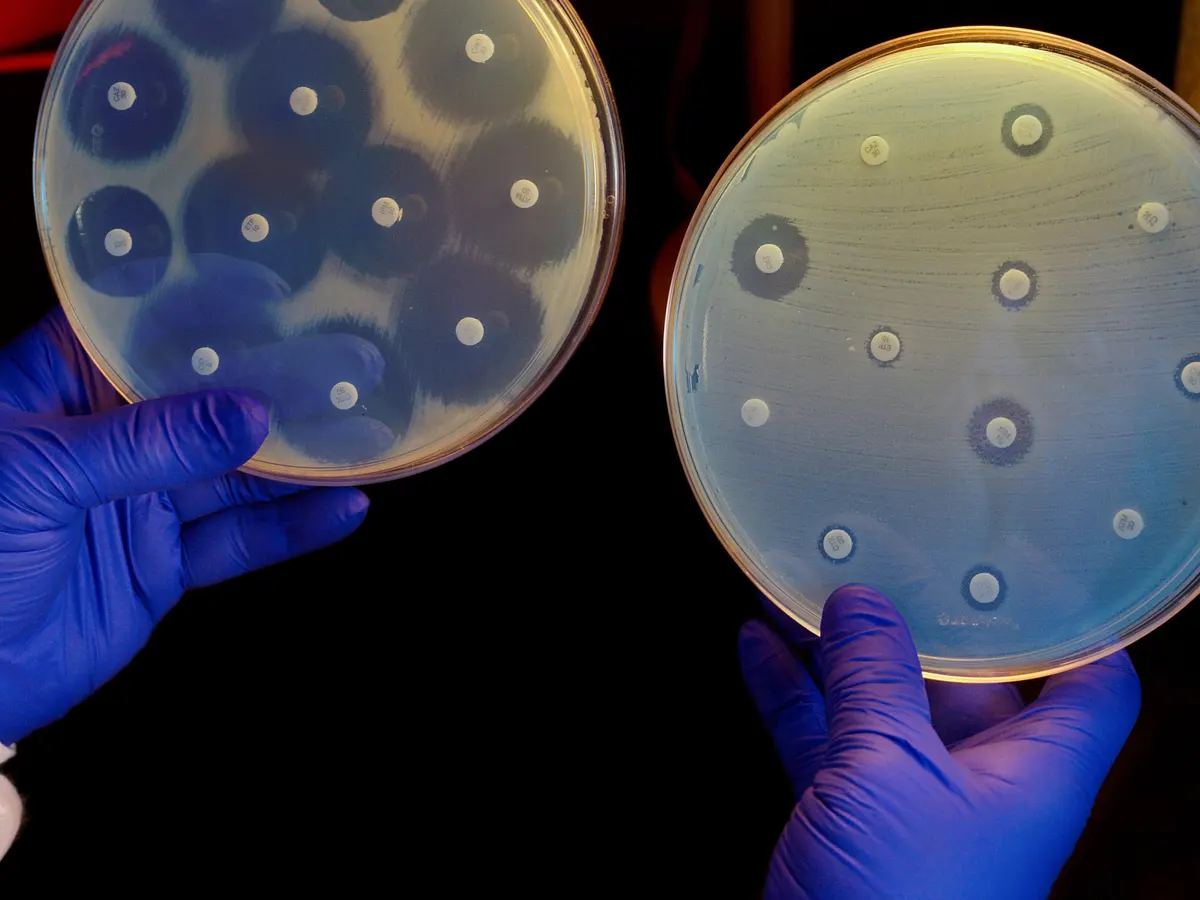
Top 3 Health Threats
The European Commission has presents a priority list of top-3 health threats that require coordination of measures at EU level in the context of medical countermeasures. The list which includes three threat categories of life-threatening or otherwise seriously harmful hazards to health which have the potential of spreading across Member States. They are: (1) pathogens with…
Europe’s Beating Cancer Plan, One Year On
The EU says another step has been made to increase access to cancer prevention, early detection, treatment and care. Ahead of World Cancer Day and a year after the publication of Europe’s Beating Cancer Plan, the Commission is launching a series of new initiatives, announced at the event “Ensuring Equal Access for All: Cancer in…
Titanium Dioxide Banned As Food Additive
The European Commission has adopted a ban on the use of Titanium Dioxide as a food additive (E171). The ban will apply after a six-month transitory period. This means that, as from this summer, this additive should no longer be added to food products. Commissioner Stella Kyriakides, in charge of Health and Food Safety, said: “The safety of the…
EU Authorises New Vaccine Nuvaxovid
The European Commission has granted a conditional marketing authorisation (CMA) for the COVID‑19 vaccine Nuvaxovid, developed by Novavax, the fifth COVID-19 vaccine authorised in the EU. This authorisation follows a positive scientific recommendation based on a thorough assessment of the safety, effectiveness and quality of the vaccine by the European Medicines Agency (EMA) and is…
EU Digital COVID 19 Certificate
The Commission has adopted rules relating to the EU Digital COVID Certificate, establishing a binding acceptance period of 9 months (precisely 270 days) of vaccination certificates for the purposes of intra-EU travel. A clear and uniform acceptance period for vaccination certificates will guarantee that travel measures continue to be coordinated, as called for by the European Council…
In Vitro Diagnostic Medical Devices Regulation
The In Vitro diagnostic medical devices regulation that will apply as from 26 May 2022, can now be progressively rolled out, thanks to its adoption by the European Parliament and the Council. In the context of the COVID-19 pandemic Member States, health institutions and economic operators redeployed financial and other resources to address the unprecedented…
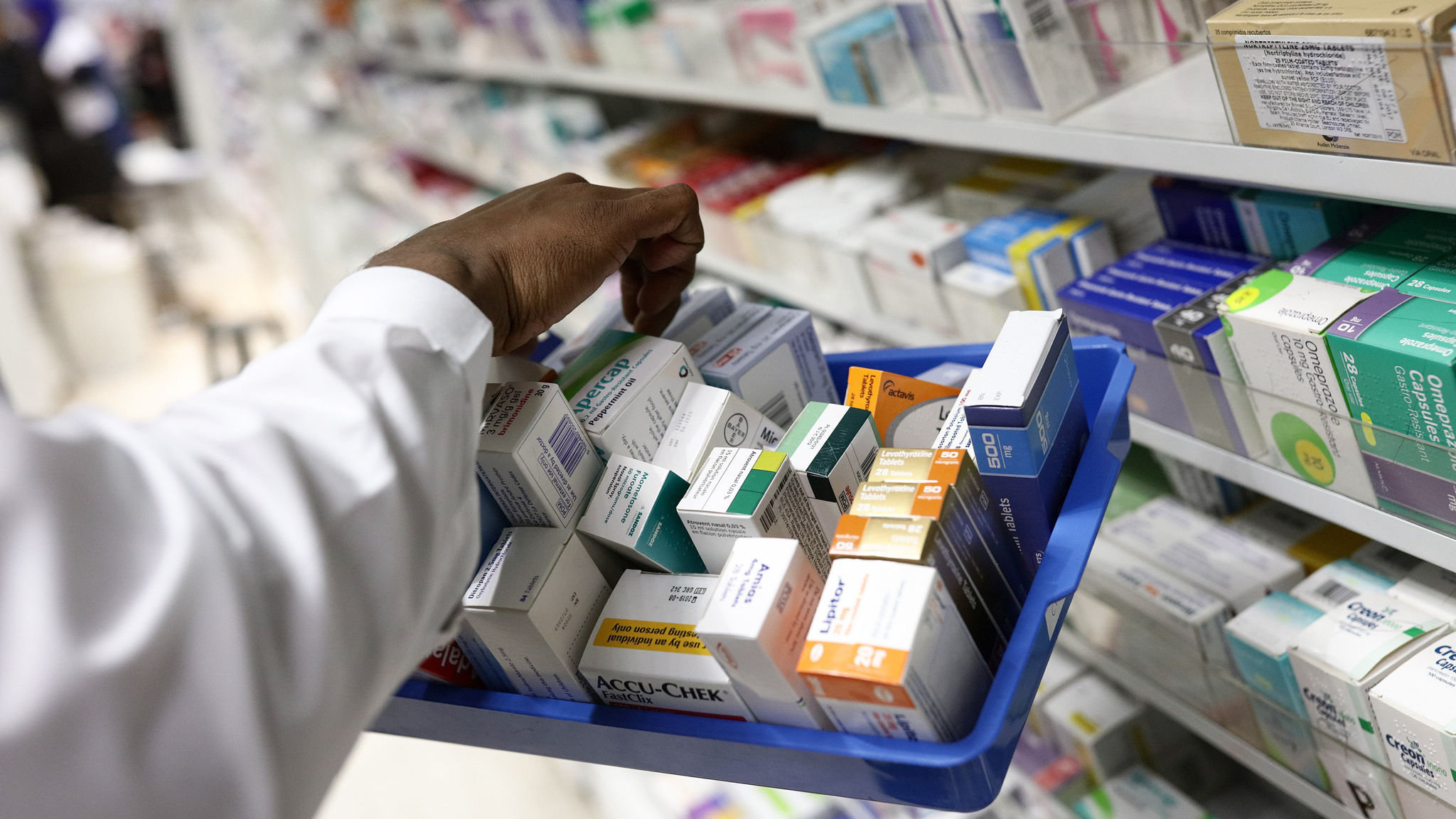
Supply of Medicines from UK
The Commission has put forward proposals to ensure the continued long-term supply of medicines from Great Britain to Northern Ireland and to address outstanding supply concerns in Cyprus, Ireland and Malta. In the context of the Protocol on Ireland/Northern Ireland, this means that the same medicines will continue to be available in Northern Ireland at…
EU and AstraZeneca Reach Agreement on Vaccine Delivery
The EU and pharmaceutical company AstraZeneca have reached an agreement which will secure the delivery of the remaining COVID-19 vaccine doses to Member States under the terms of the Advance Purchase Agreement concluded on 27 August 2020 with AstraZeneca. The agreement will also end the pending litigation before the Brussels Court. Commissioner for Health and…