Good news for health professionals engaged in the battle to halt the spread of coronavirus.
Morocco has designed the first 100% Moroccan diagnostic kit for Covid-19, tested and validated by national agencies and the world renowned Pasteur Institute in Paris.
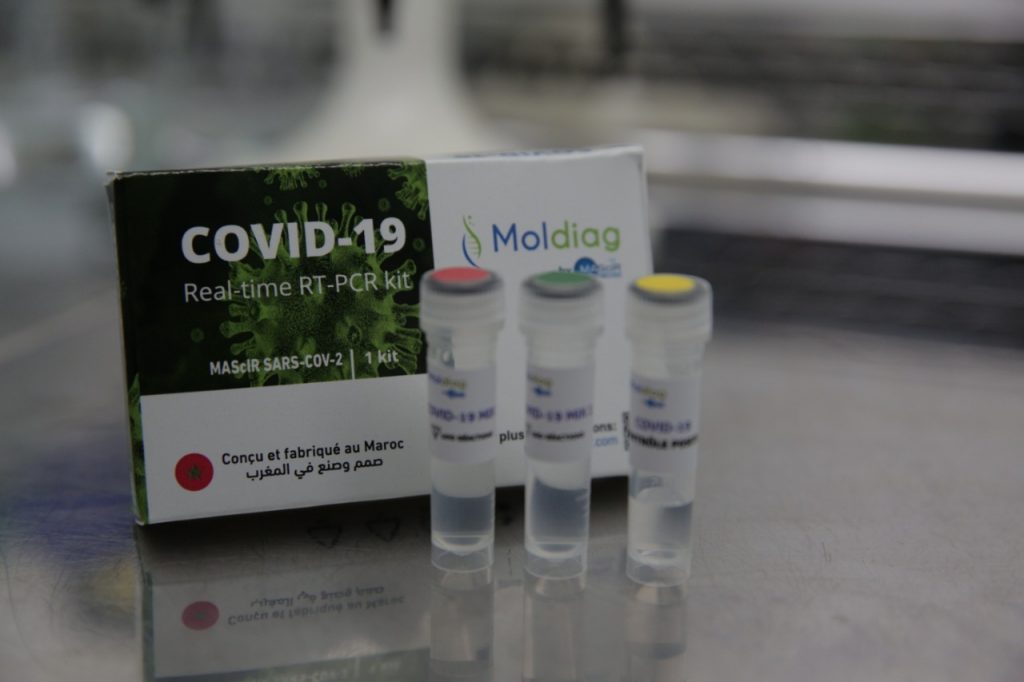
The kit has been designed by the MAScIR Foundation (Moroccan Foundation for advanced science, innovation and research), a research and development institution based in the capital Rabat.
Since the emergence of the COVID-19 pandemic and the rising number of cases in Morocco, (MAScIR) responded rapidly to the health crisis by drawing on its experience in developing molecular diagnosis kits in order to produce the first Moroccan test kit for Covid-19 disease. The diagnostic test they have developed has a high degree of sensitivity and reliability, and at a controlled cost to enable clinical and public health laboratories to quickly diagnose COVID-19.
This kit is based on the real time RT–PCR technique (real time reverse transcription–polymerase chain reaction), one of the most accurate laboratory methods for detecting, tracking and studying the COVID-19 coronavirus. In addition, this technique is highly sensitive and specific and can deliver a reliable diagnosis in 45 minutes. Also, it has a lower potential for contamination or errors, as the entire process can be carried out within a closed tube. The kit contains both the reagents and controls required for the real-time RT-PCR detection of RNA from the SARS-CoV-2 virus.
The MAScIR SARS-CoV-2 prototype kit was tested, validated on 450 clinical specimens and was approved for in vitro diagnostic use. The validation process was done in several national laboratories such as the CHU Ibn Rochd Casablanca (Centre Hospitalier Universitaire), the HMIMV- Rabat (Hôpital militaire d’instruction Mohammed V), the virology laboratories of military hospitals of Moulay Ismail in Meknès and Avicenne in Marrakech, the LRAM (Laboratoire de recherche et d’analyses biomédicales) of “la Fraternelle de la Gendarmerie Royale” in Rabat, as well as internationally, by the Institut Pasteur in Paris.
The Medical Biotechnology centre of MAScIR, where the Moroccan COVID-19 test Kit has been developed, has more than ten years of experience in the development of such molecular diagnostic kits. Its leading expertise allows it to develop several diagnostic kits targeting emerging diseases in Morocco, including cancer and infectious diseases, for example Her2 breast cancer, Chronic myelogenous leukemia (CML) (both validated nationally and internationally), prostate cancer (ongoing validation), hepatitis C (ongoing validation) and the rapid diagnosis of tuberculosis using the Loop-mediated isothermal amplification (LAMP) technique (validated nationally).
The MAScIR Foundation is a non-profit association created in 2007. It aims to promote and develop technological research centres in the fields of materials and nanomaterials, biotechnology, microelectronics and life sciences. Its work is oriented towards applied research and innovation to meet market needs.
MAScIR has cutting-edge scientific platforms and a highly qualified reservoir of human talent. Its researchers operate in different innovative and complementary fields. From mines to renewable energies, health and transport, the research carried out at MAScIR focuses on the current and future needs of industry, agriculture and other economic sectors.
The MAScIR Foundation also aims at promoting the results of its research and its invention by registering patents, through technology transfer, the creation of spin-offs and start-ups in order to contribute to the emergence in Morocco of a knowledge-based economy.
This has enabled it in the space of twelve years to file 180 patents with extensions at the African regional level. It has also published 650 scientific articles in journals of international renown and carried out more than a hundred projects with national and foreign manufacturers.
I spoke to the R&D project manager Dr. Abdeladim Moumen, who explained how the kit works: “The MAScIR test is based on a technique called real time PCR which is performed on the viral RNA genome extracted from the patient’s sample. Therefore, we need first to extract the viral RNA from the patient’s samples, this RNA molecule will then be converted to a DNA (reverse transcription) that will be amplified using PCR. Both the reverse transcription and the PCR processes are performed in a one-step system provided by the MAScIR kit. This test must be performed by laboratory professionals.”
“The whole process from RNA extraction till amplification will take approximatively one and half hours to be performed,” Dr Moumen went on to say.
When asked about the main benefits and properties of the MAScIR kit compared with other products, Dr Moumen replied, “MAScIR COVID-19 kit was clinically validated and approved in several Moroccan reference clinical laboratories such as the Royal Moroccan Armed forces-linked laboratories, (military hospitals, the Gendarmerie Royale Biosafety laboratory) and ministry of health-related laboratories : the CHU Ibn Rochd Casablanca (Centre Hôspitalier Universitaire) and INH – Rabat (Institut National d’Hygiène), as well as the world renowned Pasteur Institute in Paris. All these validations have demonstrated the high performance of our test in terms of sensitivity, specificity and accuracy compared to other commercial products currently in use.”
“At present, the production of kits is oriented towards the needs of the Moroccan domestic market, but when Morocco can reach self-sufficiency, we will then be able to think about exporting these products.”




