People recovering from cancer and other types of surgery are being urged to consider a “simple, tried-and-true” remedy …. chewing gum. Leading physician Andrew J. Vorenberg says “space-age technology” can help but so too can something like gum. “That”, he says, “is because the chewing reflex stimulates the gut and gets the digestive tract moving again,…
Healthcare
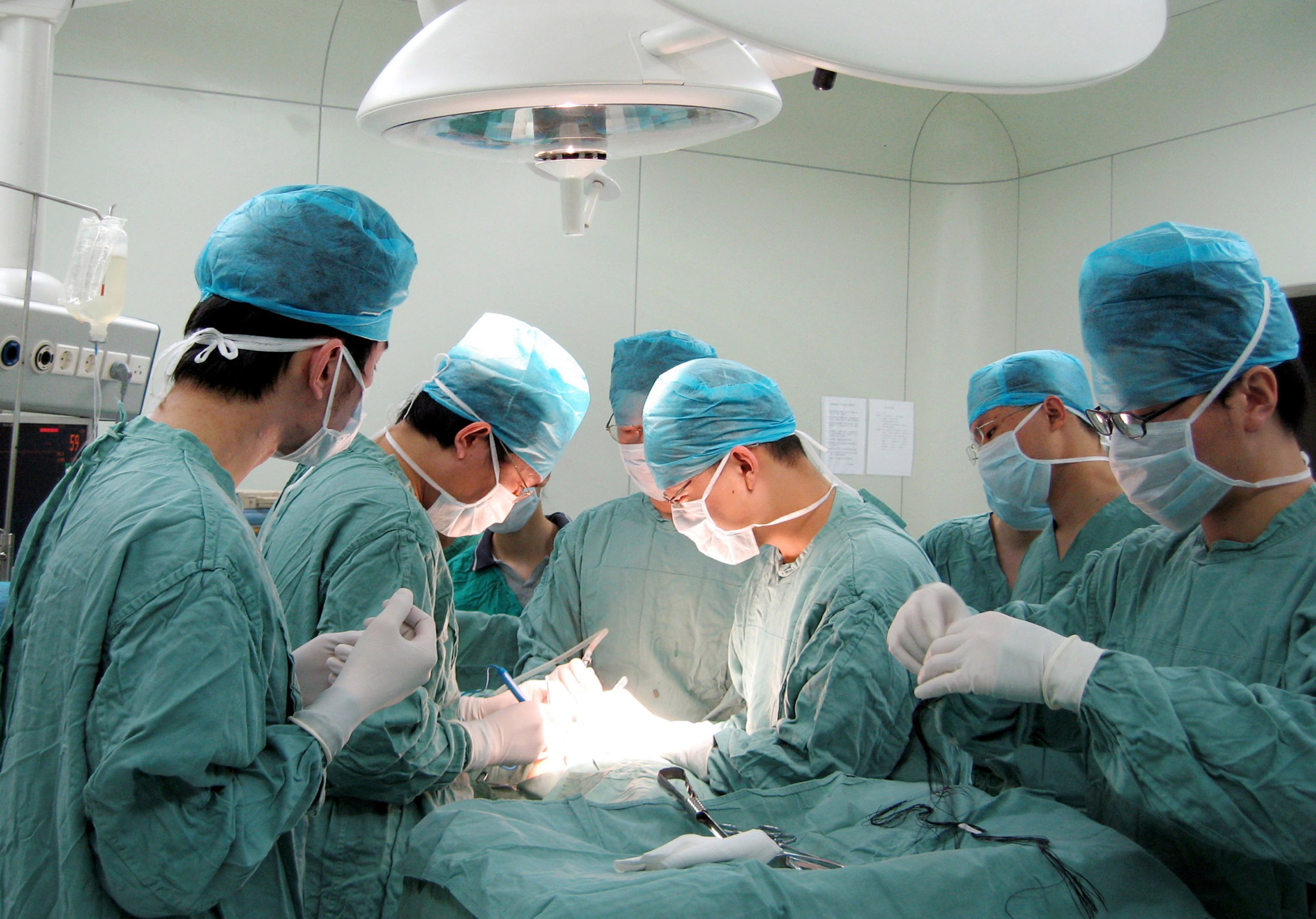
China’s Illegal Organ Harvesting on an Industrial Scale
The head of a respected rights group has called for fresh action to tackle the plight faced by Falun Gong practitioners who he says are “in the eye of Communist cyclone.” Willy Fautre, founder and director of Brussels-based Human Rights Without Frontiers was speaking at an online event in Brussels on Wednesday on the current…
Pesticide Glyphosate May Cause Cancer#HEAL,
A new report shows that scientific evidence proving that glyphosate is carcinogenic has so far been dismissed in the EU scientific assessment that will form the basis for the re-approval discussion of its EU market license. The “serious scientific shortcomings and distortions in the interpretation of EU and international scientific standards” highlighted in the report…
China Forcing Organ Removal From Prisoners
Reported systematic forced organ harvesting in China is a serious human rights violation that requires a full, independent and transparent investigation, underlined the S&Ds in regard to last week’s adoption of the European Parliament resolution on this matter. Maria Arena, the S&D negotiator on the resolution on the reports of continued organ harvesting in China, said:…
Lessons From The Pandemic
The European Parliament’s special COVI committee has elected Kathleen Van Brempt as its Chair, alongside four Vice-Chairs, and will commence its review of the EU’s response to the pandemic. Specifically tasked with looking into how the European response to the pandemic and the lessons learned can contribute to future action in a variety of EU…
EU Strategy to Fight Cancer
The European Parliament has adopted its final recommendations for a comprehensive and coordinated EU strategy to fight cancer. The report by Parliament’s Special Committee on Beating Cancer (BECA) was adopted with 652 votes in favour, 15 against and 27 abstentions on Wednesday. As more than 40% of all cancers are preventable through “coordinated actions targeting…
Commission Purchase of COVID-19 Vaccines
Early this year, the Commission announced it will buy 1.8 billion additional Pfizer Covid-19 vaccine doses. This made Pfizer the EU’s most important vendor. The deal was clinched via calls and text messages between the company’s CEO and the president of the European Commission. Left group Co-President Manon Aubry is seeking to put on the agenda of…
ICAN Campaign to Ban Nuclear Weapons
Lack of medical care and beds, overwhelmed hospitals and millions of unattended injured with serious injuries: this is the grim reality of what world capitals would face amidst a nuclear denotation, according to new report by the International Campaign to Ban Nuclear Weapons – ICAN. The new report evaluates the immediate health response capacity to…
Europe’s Beating Cancer Plan, One Year On
The EU says another step has been made to increase access to cancer prevention, early detection, treatment and care. Ahead of World Cancer Day and a year after the publication of Europe’s Beating Cancer Plan, the Commission is launching a series of new initiatives, announced at the event “Ensuring Equal Access for All: Cancer in…
Cleaning up Our Water
Drinking water across the EU will have to be monitored more closely for the potential presence of two endocrine disrupting compounds (beta-estradiol and nonylphenol) throughout the whole water supply chain. This comes after a decision by the EU on Wednesday. As required by EU rules in force since last year, the Commission established today a first ‘watch list’ of emerging compounds to…